Electroplating:
Electroplating uses the principle of electrolysis to apply a thin coat of one metal to another metal. Some practical applications include the tinplating of steel, silver-plating of nickel alloys and chromium-plating of steel. If two copper electrodes connected to a battery are placed in a beaker containing copper sulphate as the electrolyte it is found that the cathode (i.e. the electrode connected to the negative terminal of the battery) gains copper
whilst the anode loses copper.
The simple cell:
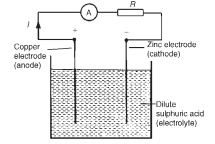
The purpose of an electric cell is to convert chemical energy into electrical energy. A simple cell comprises two dissimilar conductors (electrodes) in an electrolyte. Such a cell is shown in the figure below, comprising copper and zinc electrodes. An electric current is found to flow between the electrodes.
Other possible electrode pairs exist, including zinc-lead and zinc-iron. The electrode potential (i.e. the p.d. measured between the electrodes) varies for each pair of metals. By knowing the e.m.f. of each metal with respect to some standard electrode the e.m.f. of any pair of metals may be determined. The standard used is the hydrogen electrode. The electrochemical series is a way of listing elements in order of electrical potential, and the below given table shows a number of elements in such a series.
TABLE : Part of the electrochemical series
Potassium
sodium
aluminium
zinc
iron
lead
hydrogen
copper
silver
carbon
In a simple cell two faults exist — those due to polarization and local action.
Polarization:
If the simple cell shown in the above Figure is left connected for some time, the current I decreases fairly rapidly. This is because of the formation of a film of hydrogen bubbles on the copper anode. This effect is known as the polarization of the cell. The hydrogen prevents full contact between the copper electrode and the electrolyte and this increases the internal resistance of the cell. The effect can be overcome by using a chemical depolarizing agent or depolarizer, such as potassium dichromate which removes the hydrogen bubbles as they form. This allows the cell to deliver
a steady current.
Local action:
When commercial zinc is placed in dilute sulphuric acid, hydrogen gas is liberated from it and the zinc dissolves. The reason for this is that impurities, such as traces of iron, are present in the zinc which set up small primary cells with the zinc. These small cells are short-circuited by the electrolyte, with the result that localized currents flow causing corrosion. This action is known as local action of the cell. This may be prevented by rubbing a small amount of mercury on the zinc surface, which forms a protective layer on the surface of the electrode.
When two metals are used in a simple cell the electrochemical series may be used to predict the behaviour of the cell:
(i) The metal that is higher in the series acts as the negative electrode, and vice-versa. For example, the zinc electrode in the cell shown in the above figure is negative and the copper electrode is positive.
(ii) The greater the separation in the series between the two metals the greater is the e.m.f. produced by the cell.
The electrochemical series is representative of the order of reactivity of the metals and their compounds:
(i) The higher metals in the series react more readily with oxygen and vice-versa.
(ii) When two metal electrodes are used in a simple cell the one that is higher in the series tends to dissolve in the electrolyte.
No comments:
Post a Comment